|
|
 Nonlinear spectroscopy of bound chromophores on peptides
Nonlinear spectroscopy of bound chromophores on peptides
_k.jpg)
|
Preface:
Interdisciplinary cooperation with the Plant Physiology group at the Institute for Biochemistry and Biology:
Topics:
- Investigation of electron and energy transfer processes in pigments, e.g. the light-harvesting complexes
of the plant photosynthetic apparatus by use of transient spectroscopy
- Our plans: Exploring changes in the 3D-structure of proteins, i.e. conformational changes and folding
processes and states with ESA techniques.
Motivation: Changes in conformation are essential processes in nature, e.g. in enzymatic reactions.
Misfolding of proteins can lead to aggregations as e.g. in the prion protein. The native fold is a
prerequisite for a functional interaction with other proteins.
|
Results:
The model system of the protein myoglobin (red muscle dye, oxygen carrier, see ribbon model) and the
fluorescent dye fluorescein isothiocyanate (FITC) was synthesized, purified and characterized by different
methods.
Synthesis: The conjugation products were chemically heterogeneous with respect to their number and location
of fluorophore per/on the protein (as shown by UV/VIS spectroscopy, HPLC, MALDI-TOF mass spectrometry).
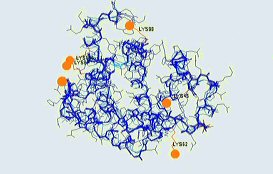
Preferred binding sites were the lysine residues 45, 62, 87, 98, 145 and the N-terminus (see backbone
picture).
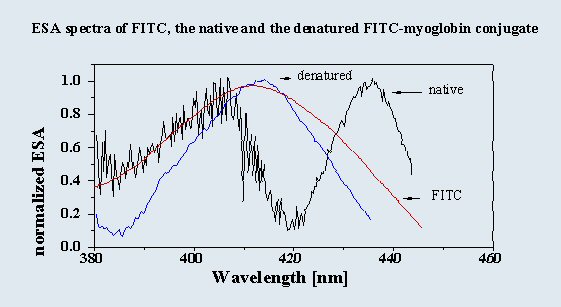
ESA spectroscopy: ESA spectra of native conjugates exhibit two maxima (around 405 and 435 nm). Both the
unbound fluorophore and the denatured conjugate have a single maximum at 410 nm (see graph). Thus, in the
native conjugate the population of bound fluorophore is also altered photophysically, and is also heterogeneous
as shown by comparison with fluorescence decay data.
|
|
| |
|
|
Conclusion:
Extrinsic fluorophores are suitable reporters of their protein environment. This is reflected in their ESA
spectra.
|
|
|
|
|
|
Sekretariat
|
|



 German
German


